
Eversense E3 CGM Approved for Two Sensors per Year: Your “Happily Ever(sense) After” - Taking Control Of Your Diabetes®
/assets/images/provider/photos/2654529.jpg)
Eversense E3 CGM: An Innovative Leap in Diabetes Management | Endocrinology & Psychiatry located in Houston and Katy, TX | Endocrine and Psychiatry Center




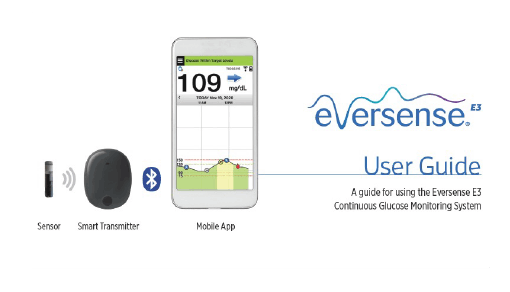





.jpg)



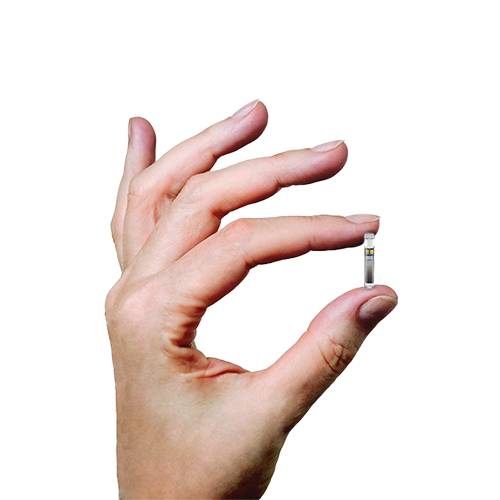


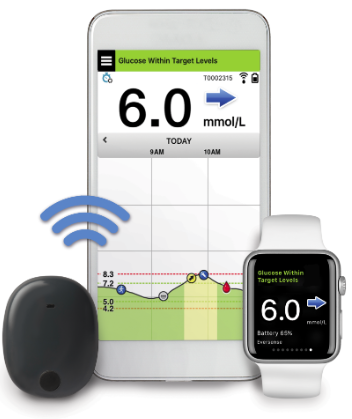


![6-Month Glucose Implant Sensor Receives CE Mark Approval [video]|Health Tech Insider 6-Month Glucose Implant Sensor Receives CE Mark Approval [video]|Health Tech Insider](https://i0.wp.com/healthtechinsider.com/wp-content/uploads/Eversense_CGM_with_video_600x275.jpg?fit=600%2C276&ssl=1)

