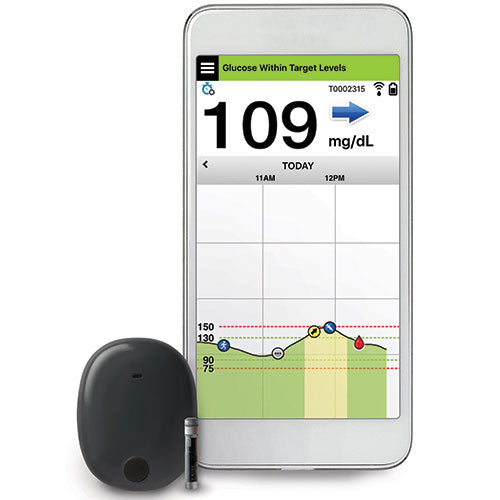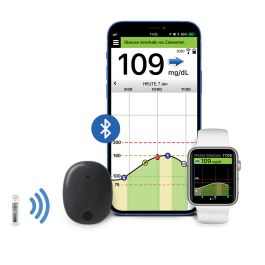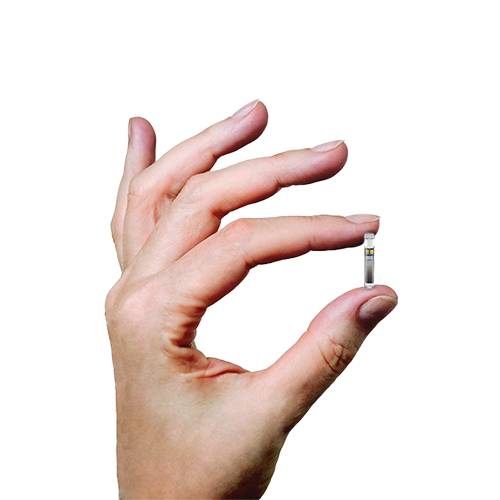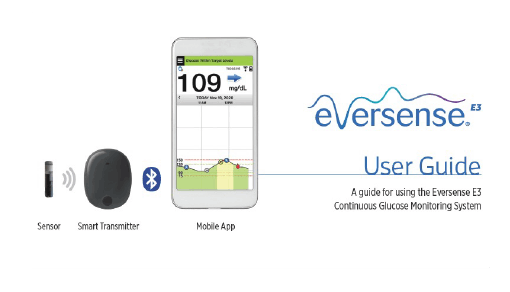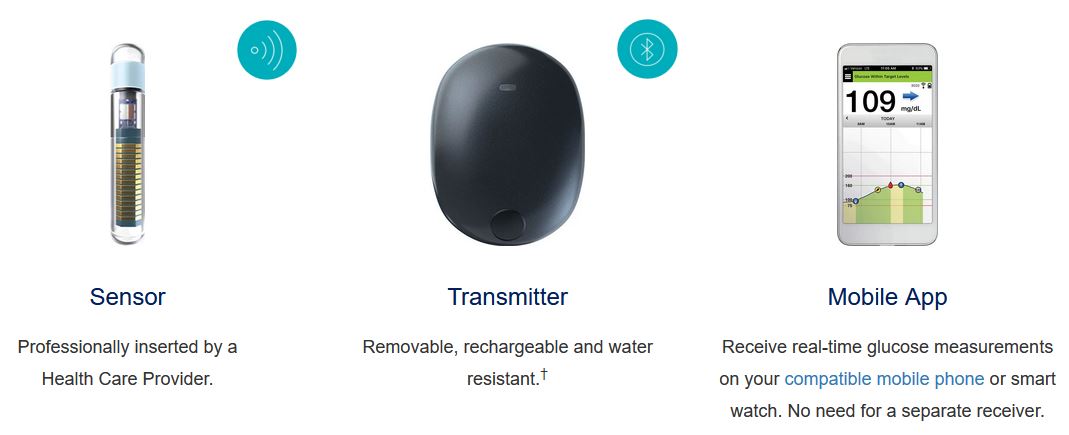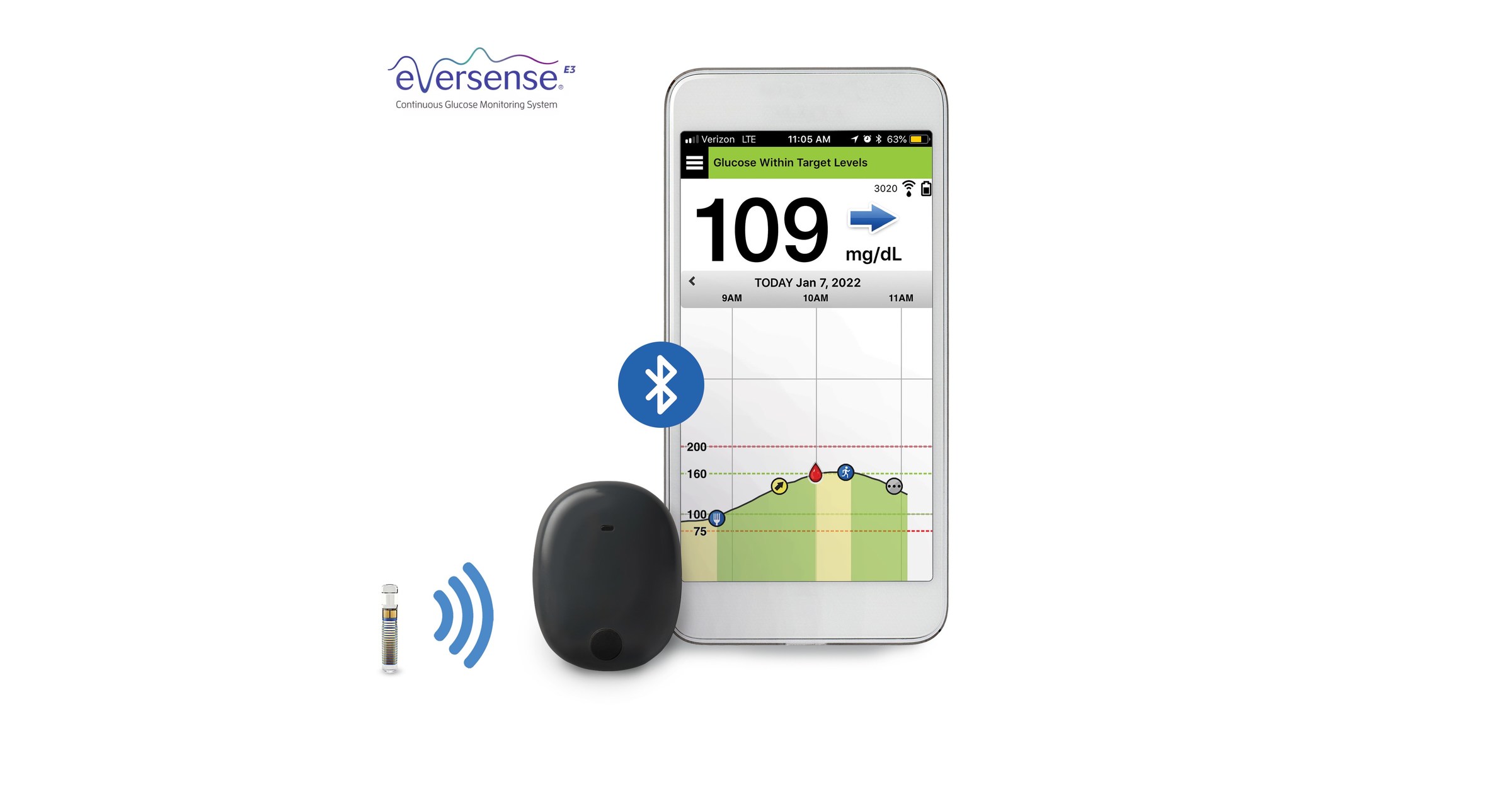
ASCENSIA DIABETES CARE LAUNCHES THE 6 MONTH EVERSENSE® E3 CONTINUOUS GLUCOSE MONITORING SYSTEM IN THE U.S. WITH THE NEW EVERSENSE PASS SAVINGS PROGRAM
Break free from frequent and sometimes painful self-insertions weekly or bi-weekly. The Eversense sensor is carefully placed under the skin by a trained health care provider and lasts up to 6 months.


.jpg)